

The presence of an arrhythmia is confirmed when the heart beats at an irregular rate. Persistent arrhythmia necessitates clinical evaluation and may require some form of intervention to prevent the irregularity from becoming life-threatening. When left untreated, serious forms of arrhythmia can lead to serious complications, such as stroke and heart failure. Recurring arrhythmia can be managed with a number of therapeutic options, including pharmaceuticals, implantable devices (e.g. pacemakers), and ablative procedures.
Catheter-based EP Products for Targeted Arrhythmia Management - Quick Summary
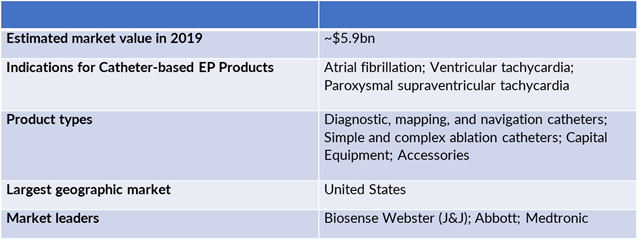
Note: EP = electrophysiology.
According to a new report from Life Science Intelligence (LSI) and Health Research International (HRI), global sales of catheter-based electrophysiology (EP) products were estimated to total $5.9bn in 2019. During the forecast period covered by this analysis, sales attributable to these products are anticipated to increase at a compound annual growth rate (CAGR) of 11.3%, reaching $10.1bn by 2024. By major product type, the catheters product segment accounts for the majority (~56%) of global catheter-based EP product sales in 2019 – a trend that is projected to continue throughout the forecast period. Within the EP catheters product segment, complex ablation catheters account for the largest share of total product sales.
Image from Boston Scientific
By geography, the US is the largest market in terms of total catheter-based EP product sales and will continue to account for the greatest share of total product sales throughout the forecast period covered by this analysis. HRI estimates that the US accounted for approximately 46% of global sales in 2019 – a trend that is projected to persist throughout the forecast.
The global market for catheter-based EP products is dominated by Biosense Webster, a subsidiary of Johnson & Johnson, and Abbott. Together these companies accounted for ~77% of global sales in 2018. Other competitors in this market include Medtronic, Boston Scientific, GE Healthcare, Stereotaxis, Stockert, and Microport Medical, among others.
Visit LSI to learn to view the report and learn more about how catheter-based EP devices are helping interventional cardiologists treat serious forms of arrhythmia. This report is available in the Medtech Pro market intelligence platform.

Schedule an exploratory call
Request InfoMarket Intelligence

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy






