
Last week, medtech startups raised approximately $82.6M in funding across 6 deals, in addition to other undisclosed deals that were announced.
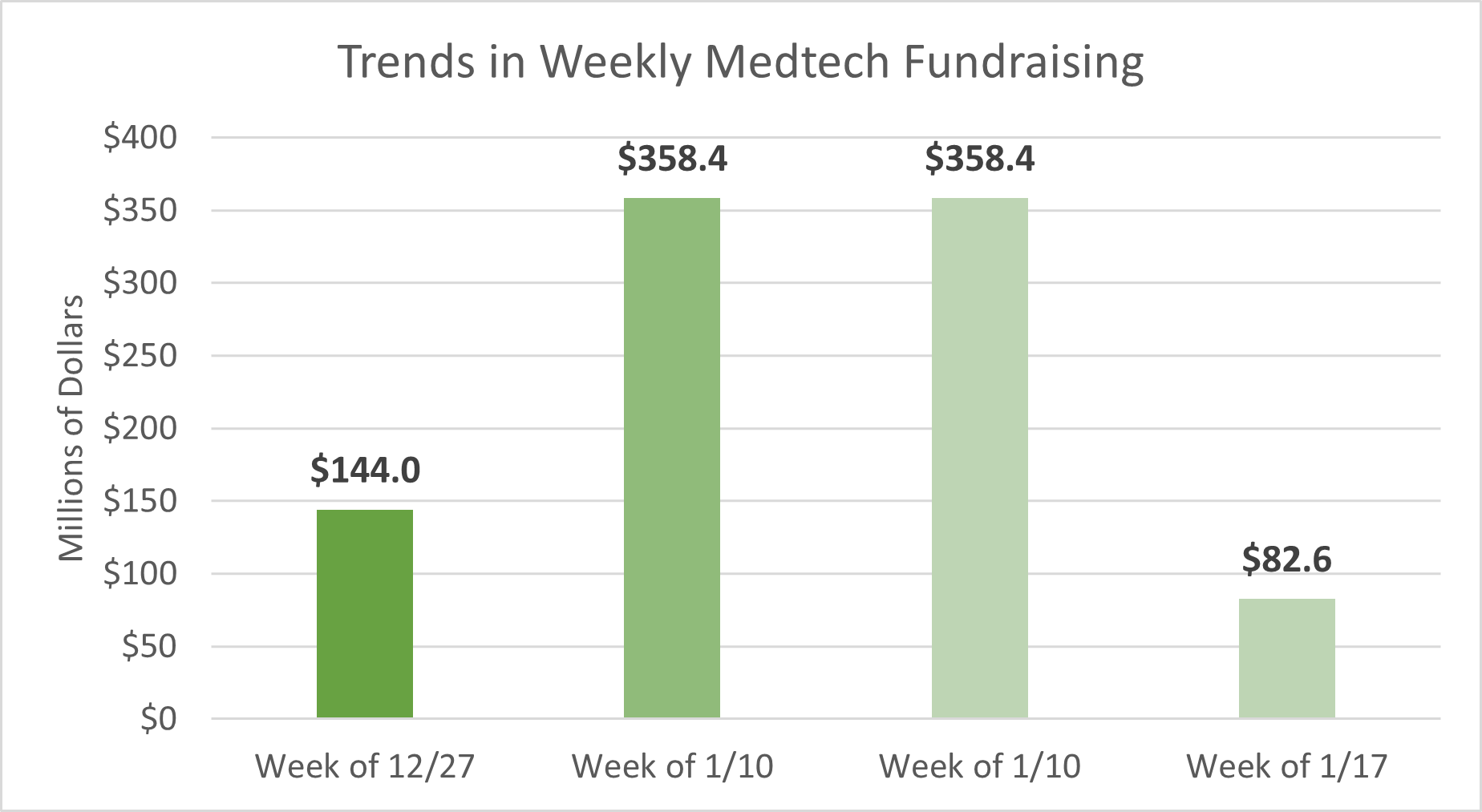
Below are some of the top deals tracked over the past week:
Start a Free Trial to Medtech Startup Tracker Today
Top Deals – Medtech Fundraising Transactions for the Week
Company | Type | Amount Raised | Round |
ProVerum Medical | Implant for the treatment of symptomatic BPH | $34,000,000 | Series A |
Chronus Health | Portable, point-of-care diagnostic system | $22,000,000 | Series A |
Aulisa | Continuous monitoring solutions for patient vitals | $13,000,000 | Series A |
Top deals for the week are based on publicly available information. At this time of this article’s publication, new deals for the week may have been announced.
LSI'sMedtech Startup Tracker is focused exclusively on covering the innovators changing the medical device landscape. Here are some of the interesting startups we’ve recently profiled:
ProVerum Medical receives $34M Series A investment
ProVerum is developing alternatives to pharmaceutical therapy for the treatment of symptomatic benign prostatic hyperplasia (BPH). The company’s first product, ProVee, is a self-expanding implant designed to reduce symptoms in patients with symptomatic BPH. ProVee distinguishes itself from other surgical treatments for BPH as an outpatient procedure which can be performed under local anesthesia in approximately 5 minutes. The ProVee device is delivered via a low-profile system similar to endoscopes which are routinely used to diagnose BPH. The Series A investment will be used to fund clinical studies to obtain regulatory approval in the US and Europe. Key strategics in the BPH treatment market includeBoston Scientific,Olympus, andTeleflex.
BrainSpace is simplifying care of neurocritical patients
External ventricular drains (EVDs) are commonly used in neurocritical patients to reduce pressure on the brain and preserve brain function. However, these devices are extremely time-consuming for nurses and healthcare professionals to manage – often requiring constant supervision to prevent injury to the patient. BrainSpace's mission is to develop solutions to improve the management of patients recovering from stroke, traumatic brain injury, and neurosurgery.
Chronus Health raises $22M in Series A funding
The investment will allow Chronus to increase its data science, engineering, and research teams, as well as fund product and technology development. Chronus is developing a truly portable point-of-care diagnostic that acquires lab results within minutes using common blood panels. The company’s system will purportedly be a low-cost, compact system that utilizes microfluidic and semiconductor technologies to enable rapid results in multiple clinical settings. Major strategics in the diagnostic testing market includeAbbott,BD, andRoche.
GrayMatters Health – digital therapeutics for mental health disorders
The digital therapeutics company is using electroencephalogram (EEG) neurofeedback to train patients to regulate patient-specific biomarkers for the treatment of mental health conditions. A patient specific biomarker for training is developed using a combination of functional MRI and EEG data that is analyzed using the company’s proprietary machine learning model. GrayMatters is initially developing its technology for the treatment of post-traumatic stress disorder. Other conditions that the company is exploring for its Prism technology include major depression disorder, borderline personality disorder, and ADHD. The company has raised $13.5M to date, having recently completed a Series A investment of $10M in January 2022.
Aulisa closes Series A round, raises $13M
According to Aulisa, the funds will be used to scale the company’s sales and marketing efforts to support continued growth of the business. Aulisa develops FDA-cleared systems that continuously measure vital signs. The company is focused on analyzing data from vital signs to provide early detection and alerts of health incidents. Aulisa’s Guardian Angel system is a wireless, wearable platform that has been adapted for local (i.e. within clinical settings) and remote use. The Guardian Angel platform is available in configurations for infant, pediatric, and adult use.
Bluegrass Vascular Technologies – innovating vascular access procedures
The company’s lead product, Surfacer, allows physicians to perform the Inside-Out procedure to obtain right-sided central venous access as an alternative to using femoral catheter access to establish permanent arteriovenous access in patients requiring the placement of a central venous catheter. The device is designed to facilitate central venous access in patients with venous obstruction. The Surfacer system has received De Novo clearance from the US FDA and the CE mark in Europe. Bluegrass is partnered withMerit Medical to distribute its Surfacer system in Europe and North America.
Life Science Intelligence is proud to announce Bluegrass Vascular Technologies & BrainSpace will be presenting companies at theEmerging Medtech Summit in March 2022.
Learn more about these medtech startups and other innovative funded startups developing innovative medical technologies using Life Science Intelligence’sMedtech Startup Tracker.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy
Subscription Includes
Global Medtech Market Analysis & Projections (MAP), 2021-2031 Published:
2023 Next Update:
Q4 2024 Deliverables:



2023
Q4 2024
Global Surgical Procedure Volumes Dashboard, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
United States Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Aesthetics, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Cardio, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
ENT, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
General, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Neuro, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
OB/GYN, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Ophthalmology, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Orthopedic, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Peripheral Vascular, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spine, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
SRS, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Urological, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Global Markets for Hip Replacement Implants, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Guidewires, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Atherectomy Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Electrosurgery, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Balloons & Vena Cava Filter, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Mechanical Heart Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tissue Heart Valve Replacement, 2023-2028 Published:
2023 Next Update:
Q2 2030 Deliverables:


2023
Q2 2030
Global Markets for Transcatheter Mitral Valve Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Femoral Closure, 2023-2029 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tricuspid Valve Repair, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Percutaneous Pulmonary Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Coronary Angio Guidewires & Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Oncology Ablation Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for ENT Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Cell Delivery Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Urology Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for External Pain Pumps, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Ureteral Access Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Pelvic Floor Repair, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Atrial Fibrillation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neurovascular Devices Ischemic, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neuromodulation Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Vertebroplasty Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Benign Prostation Hyperplasia Implants, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Cryoablation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Diagnostic Electrophysiology Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Hernia Repair, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for CRM Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Neurovascular Devices Hemorrhagic, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Renal Denervation, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Upper+Lower Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Peripheral Stents, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electromagnetic Navigation Systems, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for GI Endoscopy, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Hemodialysis, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Globals Markets for Cardiac Ablation, 2023-2028 Published:
Next Update:
Deliverables:
Global Markets for Atrial Septal Occlusion, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Aortic Grafts, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Interventional Cardiology Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Oncology Embolization, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Vascular Access Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Rotator Cuff Repair Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electrical Stimulation Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Wearable Monitoring Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Low Complexity Medical Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Canada Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Germany Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
France Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
U.K. Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Italy Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spain Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Poland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Netherlands Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Belgium Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Sweden Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Switzerland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Denmark Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Finland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Norway Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
China Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
India Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Japan Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Korea Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Australia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Thailand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Malaysia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Singapore Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
New Zealand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Caribbean Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Argentina Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Colombia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Chile Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Guatemala Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Dominican Republic Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Costa Rica Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Panama Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Mexico Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Brazil Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Turkey Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Russia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Africa Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024











