

Life Science Intelligence’s Weekly Medtech Pro Review provides a preview of select market data and startups covered in the full Medtech Pro Platform, which is a comprehensive market intelligence solution for medtech executives. As a preview of the types of content found on Medtech Pro, the Weekly Medtech Pro Review will cover select market data, procedure volumes, and startups.
Click here to learn more or subscribe to the full Medtech Pro platform.
Medtech Market Snapshot – Oncology Embolization Agents
Embolic agents are used to treat select types of cancer, including hepatic metastases, hepatocellular carcinoma, and renal cell carcinoma. Embolization involves the occlusion of a vessel to halt the flow of blood to a tumor. This process creates ischemic conditions within the tumor results in tumor necrosis. Embolic agents may also be embedded with chemotherapeutic agents that are intended to slow or stop the growth of a tumor. An additional benefit of directly delivering drugs to a tumor that has been occluded is that this prevents the circulation of chemotherapeutic drugs through the body and decreases their side effects.
According to market data from LSI, global sales attributable to embolic agents for cancer treatment were approximately $543.5M in 2020. The market for these products is projected to increase at a CAGR of 4.2% to $566.5M in 2021.
Products included under LSI’s assessment of the global market for embolic agents for cancer treatment include PVA particles, liquid embolic agents, and embolic microspheres.
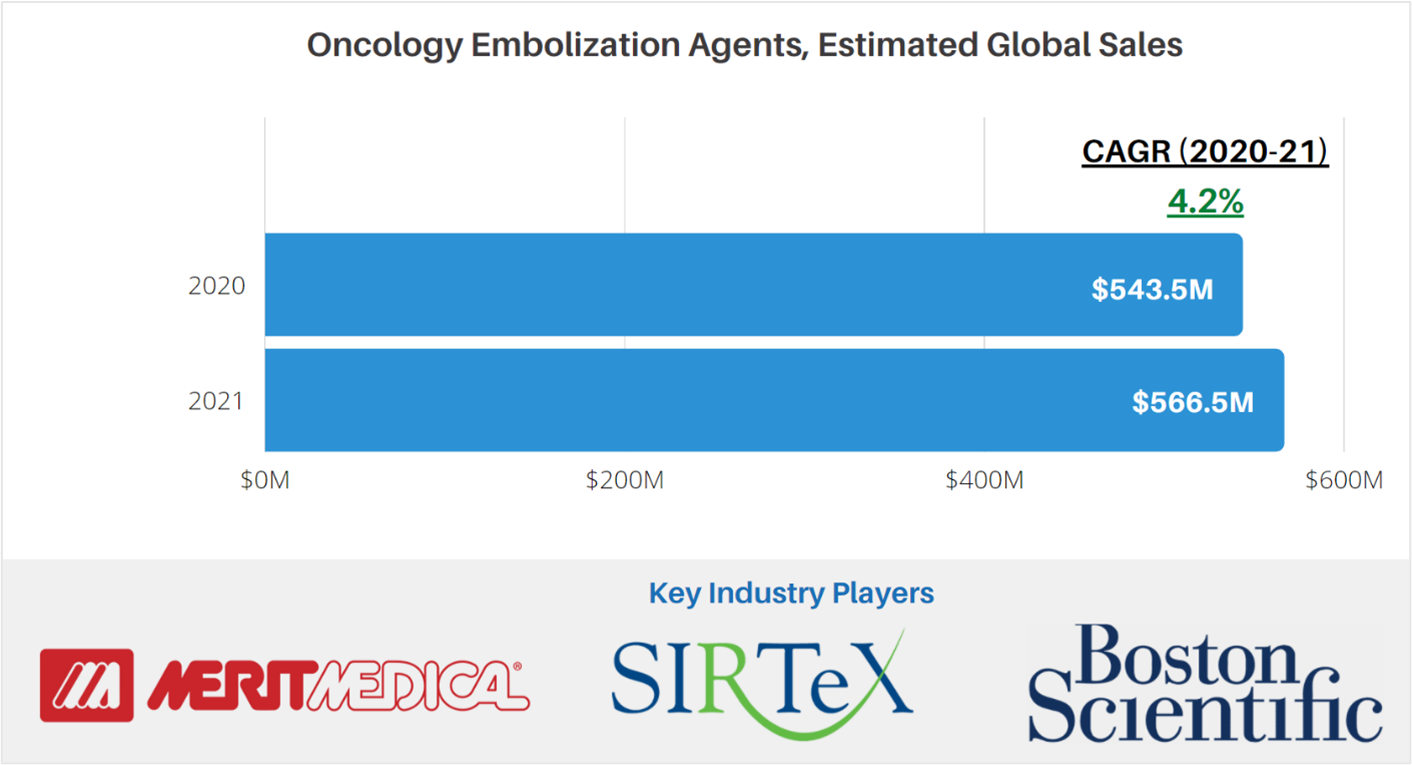
For more market data on the global oncology embolization agents market, as well as other medtech markets, visit LSI’s Medtech Pro platform.
Surgical Procedure Volumes – Heart Valve Surgeries in India
Life Science Intelligence tracks surgical procedure volumes across 37 countries and 12 major surgical markets (e.g. orthopedics, ENT, aesthetics). This week, our featured surgical procedure volume is for heart valve surgical procedures in India. Heart valve surgery is a necessary treatment for when heart valves function improperly. Valvular disease occurs when any of the valves of the heart become diseased or damaged due to factors such as infections, congenital or degenerative conditions. Depending on the severity of damage and symptoms, surgery may be used to either repair or replace the valve.
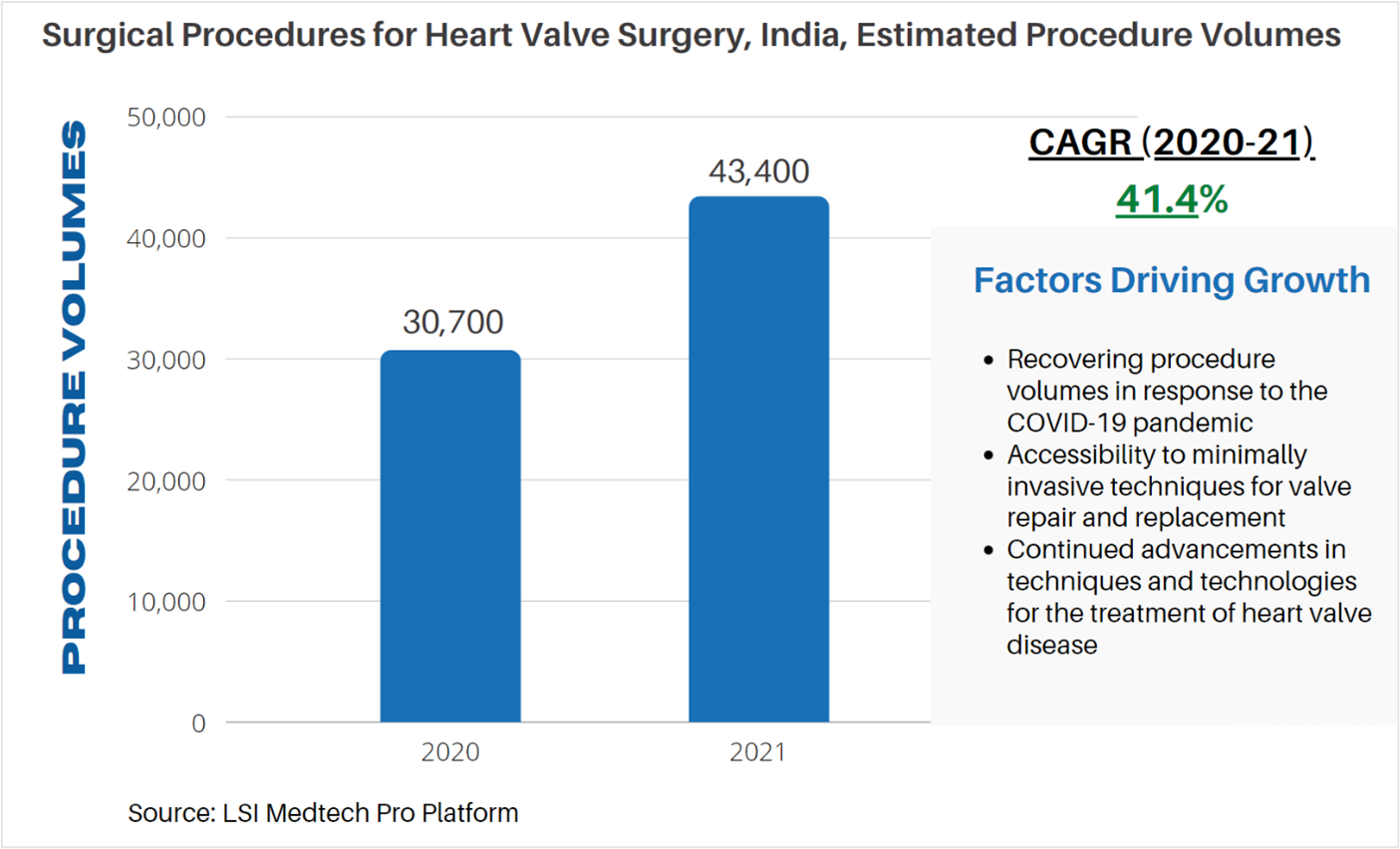
According to LSI’s Surgical Procedure Volumes database there were an estimated 30,700 valve surgeries performed in 2020 in India. The COVID-19 pandemic significantly impacted cardiac surgeries globally due to restraints on the services and resources typically required for patients receiving cardiac surgery. Due to these restraints, procedure volumes for valve surgeries were negatively impacted. While a recovery in procedure volumes has begun to take place, many countries are still navigating and adapting to COVID. In 2021, an estimated 43,400 valve repair and replacement procedures are anticipated to be performed in India.
Startup Spotlight

To see SentiAR’spresentation at the LSI Emerging Medtech Summit,click here.
SentiAR is a digital surgery startup developing augmented reality (AR) solutions for intraoperative use. The company’s flagship product CommandEP will be used to improve the success rate of the millions of cardiac ablation procedures that are performed annually.
Catheter ablation of arrhythmias is a complex and expensive procedure that requires significant resources to conduct. Despite requiring sophisticated catheter and navigation systems to perform cardiac ablation, success rates are approximately 60% after one year, with many patients requiring repeat procedures.
SentiAR is betting that its CommandEP software will improve the accuracy of cardiac ablation by allowing physicians to see a real-time model of the heart. CommandEP pulls real-time data from 3D electroanatomic mapping systems and catheters to create an anatomical and electrical hologram of a patient’s heart that “floats” above the operating space. According to CEO Berk Tas, this technology will allow physicians to focus on the procedure and reduce the cognitive load associated with managing the different technologies in the OR. The CommandEP platform currently operates on Microsoft’s Hololens hardware.
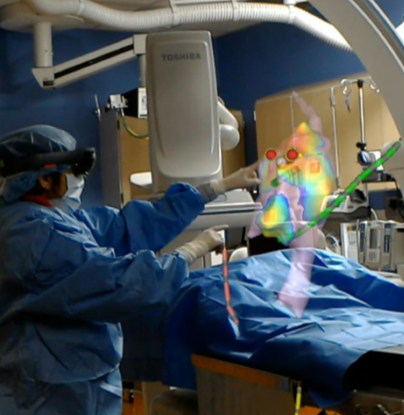
Figure 1: Example of SentiAR’s CommandEP in use
SentiAR has raised $15.1M in funding, with the most recent funding round being a Series A round that closed in April 2021.

Life Science Intelligence is proud to announce that Sense Biodetection will be a presenting at the 2022 Emerging Medtech Summit in Dana Point, California.
Healthcare has become increasingly focused on reducing costs and improving efficiency of care delivery. Sense Biodetection is helping healthcare providers achieve this with Veros – a handheld, all-in-one molecular diagnostic testing product.
Veros enables rapid PCR testing in an easy-to-use, single-use format. The platform eliminates the need for traditional hardware needed to perform PCR, bringing lab-quality results to clinicians and patients in 15 minutes. Sense Biodetection’s proclaims that their platform obtains results with just three easy steps: swab, swirl, and test. The company will initially rollout Veros for the detection of the SARS-CoV-2 virus that causes COVID-19. The platform has immediate benefits for hospitals, nursing centers, and urgent care facilities in need of instrument free point-of-care testing for COVID-19.
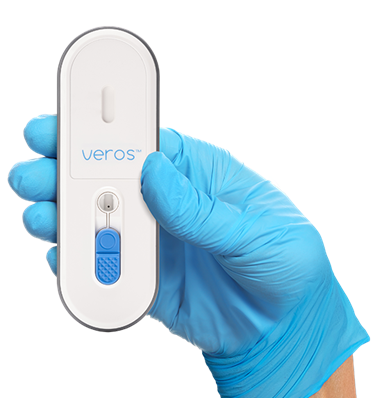
Figure 2: Sense Biodetection's Veros diagnostic
In September 2021, Sense Biodetection announced that it relocated its research and development team into a larger facility in Oxfordshire. According to Sense, the move will allow the company to accelerate the development of its testing portfolio for use with the Veros platform.
Sense Biodection has raised approximately $66.5M in total funding. In April 2021, the company closed a $50M Series B investment that will be used to advance commercialization of the Veros product and develop new products to expand the company’s portfolio of accessible, rapid molecular tests.


Preventable medical errors are a leading cause of death in the US. Founded in 2017, Activ Surgical’s mission to develop solutions that improve minimally invasive surgical systems and reduce surgical complications.
At the core of Activ’s products is ActivEdge, a surgical intelligence software powered by machine learning. ActivEdge aggregates surgical data to support intraoperative surgical decision making. The company’s first product, ActivSight, is a hardware agnostic imaging module that connects to endoscopic systems to augment intraoperative visual data. ActivSight augments traditional endoscopic imaging with near-infrared and real-time fluorescence imaging capabilities, granting surgeons new visual data that is intended to improve surgical outcomes. The device has received FDA 510(k) clearance.

Figure 3: Activ Surgical’s ActivSight Imaging Module
In June 2021, Activ Surgical announced the second product in its portfolio of digital surgery solutions – ActivInsights. This software will offer surgeons real-time tissue assessment through an AR overlay.
The company recently secured $45M in Series B funding on September 30th, 2021. The investment will support the commercialization of the company’s digital surgery suite and pursue regulatory approval in Europe as the next step in the global launch of its software platform.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy
Subscription Includes
Global Medtech Market Analysis & Projections (MAP), 2021-2031 Published:
2023 Next Update:
Q4 2024 Deliverables:



2023
Q4 2024
Global Surgical Procedure Volumes Dashboard, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
United States Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Aesthetics, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Cardio, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
ENT, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
General, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Neuro, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
OB/GYN, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Ophthalmology, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Orthopedic, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Peripheral Vascular, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spine, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
SRS, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Urological, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Global Markets for Hip Replacement Implants, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Guidewires, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Atherectomy Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Electrosurgery, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Balloons & Vena Cava Filter, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Mechanical Heart Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tissue Heart Valve Replacement, 2023-2028 Published:
2023 Next Update:
Q2 2030 Deliverables:


2023
Q2 2030
Global Markets for Transcatheter Mitral Valve Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Femoral Closure, 2023-2029 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tricuspid Valve Repair, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Percutaneous Pulmonary Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Coronary Angio Guidewires & Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Oncology Ablation Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for ENT Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Cell Delivery Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Urology Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for External Pain Pumps, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Ureteral Access Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Pelvic Floor Repair, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Atrial Fibrillation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neurovascular Devices Ischemic, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neuromodulation Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Vertebroplasty Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Benign Prostation Hyperplasia Implants, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Cryoablation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Diagnostic Electrophysiology Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Hernia Repair, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for CRM Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Neurovascular Devices Hemorrhagic, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Renal Denervation, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Upper+Lower Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Peripheral Stents, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electromagnetic Navigation Systems, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for GI Endoscopy, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Hemodialysis, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Globals Markets for Cardiac Ablation, 2023-2028 Published:
Next Update:
Deliverables:
Global Markets for Atrial Septal Occlusion, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Aortic Grafts, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Interventional Cardiology Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Oncology Embolization, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Vascular Access Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Rotator Cuff Repair Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electrical Stimulation Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Wearable Monitoring Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Low Complexity Medical Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Canada Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Germany Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
France Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
U.K. Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Italy Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spain Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Poland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Netherlands Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Belgium Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Sweden Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Switzerland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Denmark Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Finland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Norway Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
China Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
India Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Japan Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Korea Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Australia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Thailand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Malaysia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Singapore Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
New Zealand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Caribbean Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Argentina Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Colombia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Chile Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Guatemala Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Dominican Republic Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Costa Rica Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Panama Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Mexico Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Brazil Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Turkey Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Russia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Africa Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024











