

Life Science Intelligence’s Weekly Medtech Pro Review provides a preview of select market data and startups covered in the full Medtech Pro Platform, which is a comprehensive market intelligence solution for medtech executives. As a preview of the types of content found on Medtech Pro, the Weekly Medtech Pro Review will cover select market data, procedure volumes, and startups.
Click here to learn more or subscribe to the full Medtech Pro platform.
Medtech Market Snapshot – Neuromodulation Devices
Neuromodulation devices, alter the electrical signals in the body with the intention of improving function or reducing pain in the body. There are multiple types of neuromodulation devices in use today, including implantable, transcutaneous, and transcranial devices. Neuromodulation devices are often used to treat patients who have failed to respond to pharmacological management of their condition.
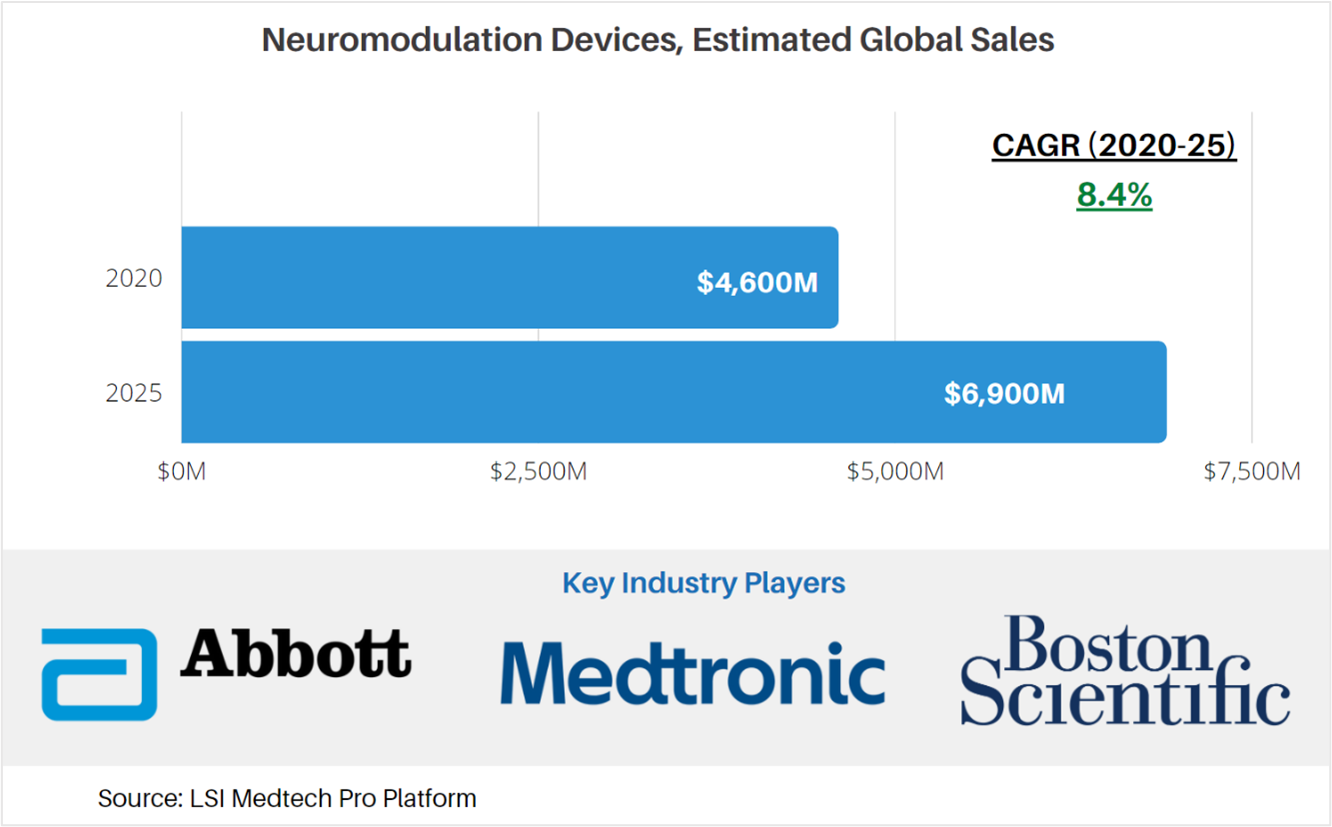
According to market data from LSI, global sales attributable to neuromodulation devices were approximately $4.6B in 2020. The market for these products is projected to increase at a CAGR of 8.4% to $6.9B in 2025. As is the case with many medtech markets, unit sales and implantation procedure volumes for neuromodulation devices were significantly impacted by the COVID-19 pandemic.
Products included in LSI’s assessment of the global neuromodulation devices market include transcutaneous and implantable devices. Implantable devices account for approximately 75% of total neuromodulation device sales.
For more market data on the global market for neuromodulation devices, as well as other medtech markets, visit LSI’s Medtech Pro platform.
Surgical Procedure Volumes – Radical Neck Dissection in the UK
Life Science Intelligence tracks surgical procedure volumes across 37 countries and 12 major surgical markets (e.g. orthopedics, ENT, aesthetics). This week, our featured surgical procedure volume is for radical neck dissections performed in the UK. Radical neck dissection is considered the “gold standard” for the treatment of head and neck cancers and metastatic lymph nodes. Neck dissection can be modified to be more conservation or radical, depending on clinical presentation of metastatic disease. Any neck dissection involves the excision of soft tissue in the neck and the lymph nodes contained therein. Modifications of radical neck dissection are done to preserve one or more non-lymphatic structures.
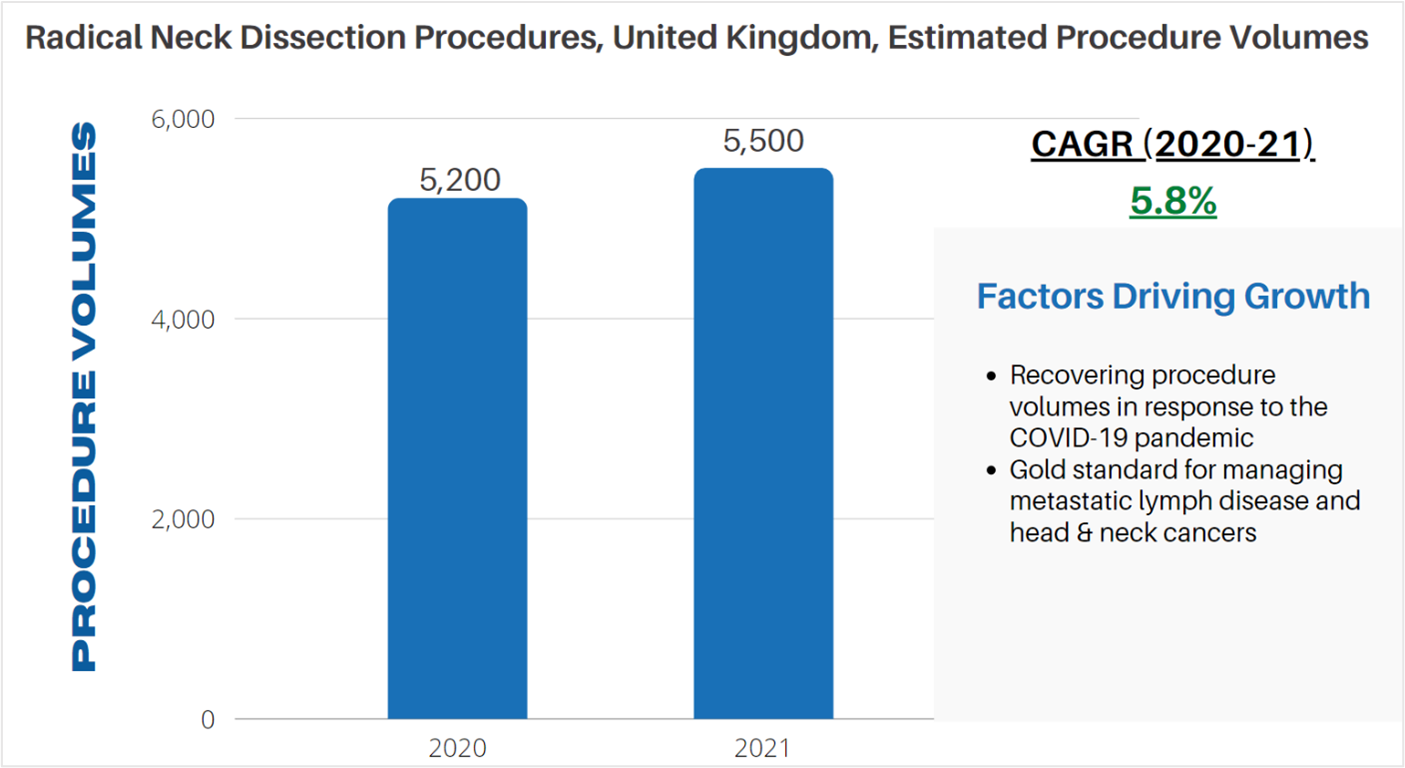
According to LSI’s Global Surgical Procedure Volumes database there were an estimated 5,200 radial neck dissections performed in 2020 in the UK. The COVID-19 pandemic had a restraining effect on radical neck dissections, largely dueto a reduction in surgical capacity and a reluctance from patients to have surgery during the height of the pandemic. A recovery in procedure volumes is projected to occur in 2021, with LSI estimating that 5,500 procedures will be performed in the UK. Following this recovery from the pandemic, radical neck dissection procedures are projected to increase in line with epidemiologic trends for head and neck cancers.
Startup Spotlight

To see AiM Medical Robotics presentation at the LSI Emerging Medtech Summit, click here.
Despite the improvements in pre-operative imaging and mapping technologies, many challenges still necessitate the need for removals during surgery and repeat revisions. To improve neurosurgical outcomes, AiM Medical Robotics is developing a robotic system that is compatible with intraoperative magnetic resonance imaging (MRI).
Licensed from technology developed at Worcester Polytechnic Institute, AiM’s robotic system is intended to be portable system that will enable real-time guidance of therapy delivery. The system will use a needle-based ultrasonic device to safely ablate tumors and structures of the brain causing functional neurological disorders (e.g. epilepsy). Current neurosurgical solutions involve the use of harmful radiation-based technologies (e.g. wide-beam radiation therapy) or lasers, which can cause damage to surrounding tissue.
The company has raised approximately $2.3M in funding. AiM Medical Robotics is planning to bring the robot to market in 2023.

Life Science Intelligence is proud to announce that Propriowill be a presenting at the 2022 Emerging Medtech Summit in Dana Point, California.
Proprio is developing a digital surgery platform to augment the capabilities of surgeons. The company’s goal is to reduce the cognitive and ergonomic load on the surgeon, while providing them with 3D images that offer more dynamic information compared to standard 2D images captured from legacy surgical imaging technologies.
The PARADIGM system is a real-time multimodal imaging device that utilizes artificial intelligence to reconstruct 2D images into 3D. The digital reconstruction is then visible to the surgeon through a wearable headset. Tracking technology in the surgeon’s headset will allow the system to give the surgeon 3D data specific to wear the surgeon is looking. Proprio’s system will provide surgeons with pre-operative imaging, virtual annotation, collaborative feedback, and improved magnification through – all within the surgeon’s field-of-view. Post-surgery, the data from procedures performed using Proprio’s technology will be available for review and teaching via simulation.
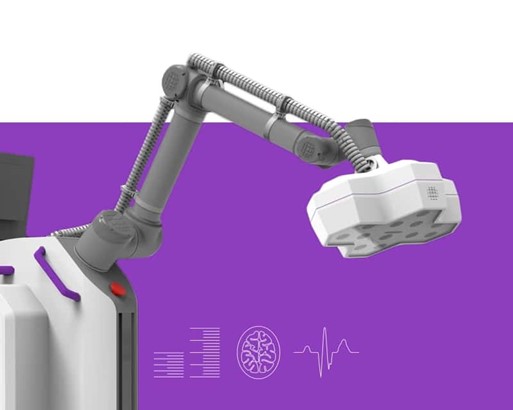
Figure 1: Proprio PARADIGM system
To date, Proprio has raised $42.1M in funding. Key investors include virtual reality hardware developer HTC and tech-giant Intel, among other investor groups.
Currently, Proprio is evaluating the use of their technology in neuro and orthopedic surgery. The company’s product is currently pending FDA clearance.

San Diego-based Biolinqis developing a pain-free solution for the measurement of blood glucose levels. While major strategics such as Abbott, Dexcom, and Medtronicoffer needle-free continuous glucose monitors (CGMs), Biolinq’s device will further miniaturize the technology that is changing the way diabetics manage their disease.
The company’s technology, which was spun out from UC San Diego, is a coin-sized patch that utilizes proprietary microarray technology to measure glucose found in the interstitial fluid between cells underneath the skin. In addition to its smaller size, Biolinq’s biosensor further distinguishes itself by not requiring a needle or applicator to place the sensor beneath the skin – the company’s technology rests on top of the capillary bed in the skin.
Biolinq plans to expand the monitoring capabilities of their biosensor, purporting that their electrochemical sensor can monitor other biomarkers.
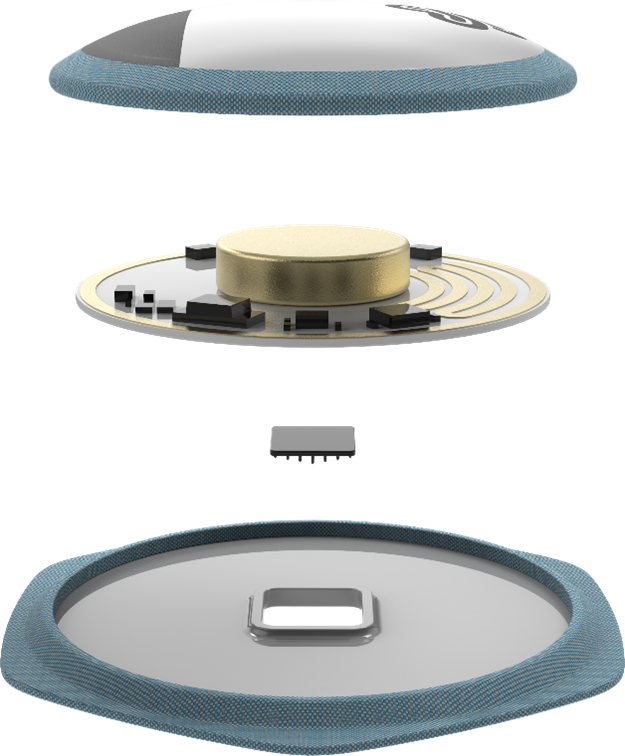
Figure 2: Biolinq glucose biosensor
In November 2021, Biolinq announced that it had secured a $100M investment from a Series B round. According to the company, the funding will help advance their technology through the regulatory review process and begin commercialization.

Schedule an exploratory call
Request Info17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy
Subscription Includes
Global Medtech Market Analysis & Projections (MAP), 2021-2031 Published:
2023 Next Update:
Q4 2024 Deliverables:



2023
Q4 2024
Global Surgical Procedure Volumes Dashboard, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
United States Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Aesthetics, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Cardio, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
ENT, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
General, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Neuro, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
OB/GYN, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Ophthalmology, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Orthopedic, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Peripheral Vascular, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spine, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
SRS, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Urological, Global Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Global Markets for Hip Replacement Implants, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Guidewires, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Atherectomy Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Electrosurgery, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Peripheral Vascular Balloons & Vena Cava Filter, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Mechanical Heart Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tissue Heart Valve Replacement, 2023-2028 Published:
2023 Next Update:
Q2 2030 Deliverables:


2023
Q2 2030
Global Markets for Transcatheter Mitral Valve Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Femoral Closure, 2023-2029 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Tricuspid Valve Repair, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Percutaneous Pulmonary Valves, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Coronary Angio Guidewires & Catheters, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Oncology Ablation Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for ENT Devices, 2023-2028 Published:
2023 Next Update:
Q2 2024 Deliverables:


2023
Q2 2024
Global Markets for Cell Delivery Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Urology Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for External Pain Pumps, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Ureteral Access Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Pelvic Floor Repair, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Atrial Fibrillation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neurovascular Devices Ischemic, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Neuromodulation Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Vertebroplasty Devices, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Benign Prostation Hyperplasia Implants, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Cryoablation, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Diagnostic Electrophysiology Catheters, 2023-2028 Published:
2023 Next Update:
Q3 2024 Deliverables:


2023
Q3 2024
Global Markets for Hernia Repair, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for CRM Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Neurovascular Devices Hemorrhagic, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Renal Denervation, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Upper+Lower Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Peripheral Stents, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electromagnetic Navigation Systems, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for GI Endoscopy, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Hemodialysis, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Globals Markets for Cardiac Ablation, 2023-2028 Published:
Next Update:
Deliverables:
Global Markets for Atrial Septal Occlusion, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Aortic Grafts, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Interventional Cardiology Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Oncology Embolization, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Vascular Access Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Rotator Cuff Repair Suture Anchors, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Electrical Stimulation Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Wearable Monitoring Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Global Markets for Low Complexity Medical Devices, 2023-2028 Published:
2023 Next Update:
Q4 2024 Deliverables:


2023
Q4 2024
Canada Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Germany Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
France Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
U.K. Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Italy Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Spain Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Poland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Netherlands Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Belgium Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Sweden Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Switzerland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Denmark Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Finland Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Norway Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
China Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
India Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Japan Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Korea Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Australia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Thailand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Malaysia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Singapore Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
New Zealand Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Caribbean Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Argentina Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Colombia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Chile Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Guatemala Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Dominican Republic Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Costa Rica Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Panama Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Mexico Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Brazil Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Turkey Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
Russia Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024
South Africa Surgical Procedure Volumes, 2018-2029 Published:
2022 Next Update:
Q2 2024 Deliverables:


2022
Q2 2024











