


| Snapshot Aspect | Data and Details |
| Base Year for Estimate | 2023 |
| Forecast Period | 2023 - 2028 |
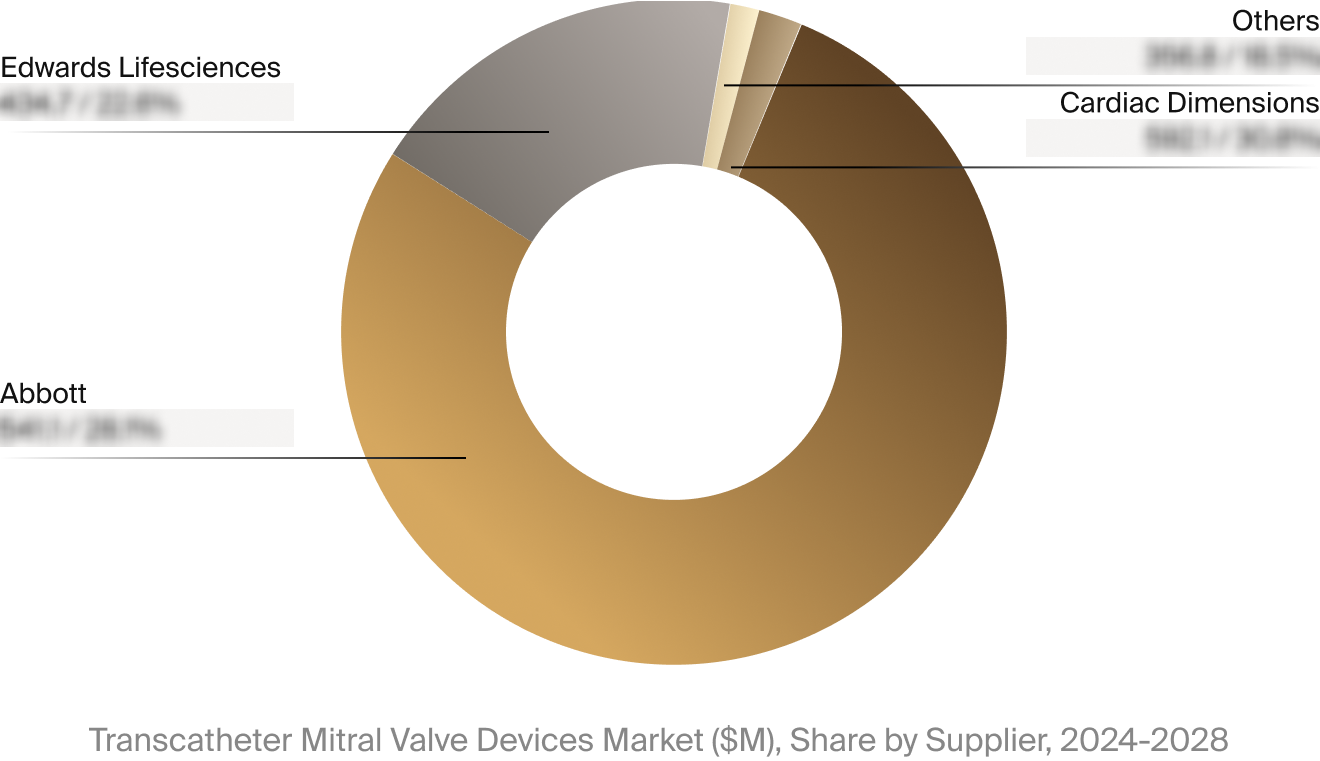
| Market Size in 2023 | $1.3 billion |
| CAGR | 23.2% |
| Projected Market Size in 2028 | $3.8 billion |
| Company | Date | Event Type | Event |
|
4C Medical
|
5/2024 | Regulatory | 4C Medical’s AltaValve™ system received FDA Breakthrough Device designation for two therapeutic indications. |
|
Edwards Lifesciences
|
7/2024 | M&A | Edwards Lifesciences announced the acquisition of Innovalve and secured a stake in Affluent Medical. |
Trusted By The Companies Pioneering What’s Next































































17011 Beach Blvd, Suite 500 Huntington Beach, CA 92647
714-847-3540© 2025 Life Science Intelligence, Inc., All Rights Reserved. | Privacy Policy